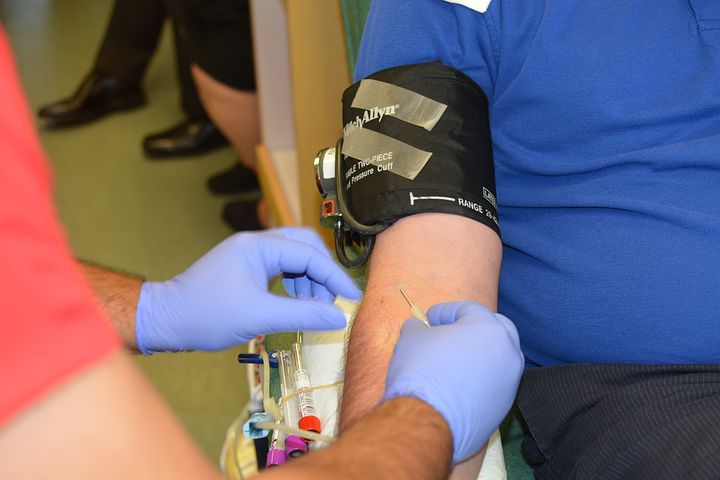
On Friday, Jan. 27, the US Food and Drug Administration announced that its proposing a change to blood donor eligibility.
The FDA is going to begin using “gender-inclusive, individual risk-based questions to reduce the risk of transfusion-transmitted HIV,” rather than relying on a person’s sexuality.
This is another step in the Food and Drug Administration’s move away from decades-old rules meant to keep HIV out of the blood supply. When the AIDs crisis hit in the early 1980s the FDA instigated a ban on blood donations from gay men. However, that’s been changing over the past decade.
Eight years ago, the FDA began allowing gay and bisexual men to donate blood if they had gone a year without having sex. Three years ago – largely in response to dramatic drops in blood donation during the COVID-19 pandemic – the FDA began allowing gay and bisexual men who hadn’t had sex in the previous three months to donate blood.
The proposed new guidelines announced on Friday would drop the requirement for gay and bisexual men in monogamous relationships to abstain from sex before donating blood.
One reason cited is an improved ability to test for the HIV virus.
Soon after the announcement by the FDA, North Carolina Department of Health and Human Services Secretary Kody Kinsley and State Health Director and Chief Medical Officer Dr. Elizabeth Cuervo Tilson weighed in on the issue, saying in a joint statement that this is “a major step forward for ending stigmatization of gay and bisexual men.”
The two wrote the following: “We applaud the life-saving decision by the US Food and Drug Administration to adapt its rules for blood donation, joining countries around the world in proposing a set of rules that defers donors for risky behaviors, not for who they are. This decision allows a previously marginalized group of people to participate in one of the most selfless acts that individuals perform, coming together to save lives.”
They went on to say that this is the best way to ensure a “safe and robust supply” of blood.
The two state health officials added that this is a very welcome development since the number of blood donations in the state has been way down since the pandemic.
Kinsley and Tilson had something to do with the FDA’s policy change. They, and health officials from nine other states and the District of Columbia, wrote a letter to the FDA in March of last year asking that the FDA’s blood donation policy be changed. The letter stated that the specificity of HIV testing now available all but eliminates the risk of the virus getting into the blood supply.
The group of health officials argued that restrictions on blood donation should be based on risky behavior – not a person’s sexuality.
The new FDA rules will go through a 60-day comment period. The FDA will review comments before finalizing its guidance.

Scott it is not THE FDA…the is a bad word now. LOL
As a paramedic, my sister had routine testing for AIDS because she was not allowed to ask and patients weren’t required to tell if they were infected. This was a concern she dealt with for over 30 years. I remember being told as a volunteer firefighter that I’d been exposed to a patient with AIDS in 1980 on an accident scene before the use of protective gear was a norm. For years I wondered if I’d be told that my blood donation was denied after testing (I waited to donate for two years after the notification). Since the recent COVID virus, I’ve questioned how the virus itself and the vaccine/boosters would affect the blood supply for people who chose to not be vaccinated and know there are countries that have started to bank the blood of unvaccinated people so there are options other than self banking of blood.
To lower the standard of blood donations so that men who engage in sex with men in a “monogamous” relationship are allowed to donate blood when it’s known that homosexual sex is the main transmitter of AIDS is a major safety risk to our blood supply. It should also be made clear that a transgender person who is male at birth and has sex with a male should not be able to give blood if they’ve had sex with another biological male in the last three (6-12 would be better) months. The safety of our blood supply is critical to the health of all of us and anyone who puts the “feelings” of a person over the safety of a major supply like blood being given to strangers is simply self-centered and evil. There’s no reason to change the standards, there is a need for the Red Cross to do more to advertise and encourage people to donate. It’s their job to find donors who feel safe to donate, understand the need and urgency, and are willing to show up to give blood. They scared people away from donations during COVID and now they need to encourage them to come back, especially young people who feel no need to do anything for the people in their community.
The ignorant statements within your comment is staggering…
Homosexual sex doesn’t transmit AIDS any more or less than hetero sex…but sexual promiscuity without protection led to prevalence of AIDS in the gay community. Monogamous gay men are as of a low a risk of AIDS as monogamous hetero couples.
COVID vaccine doesn’t stay in your blood more than a few days.
Thank goodness more highly education people are responsible for the safety of our blood supply.
Oh well Dorothy we’re not in Kansas anymore the all knowing all mighty wizard chris has spoken just like slo joe who just said this weekend “ I give my word as a biden” Americas future looks great. Man do I feel better with that statement. I’m thinking that ole joe has another son besides hunter. Welcome to our world chris biden
HIV is mainly spread through the exchange of bodily fluids.
Oh My God!
You really are an effing moron.
Supercilious too.
I’m certain Leviticus & Romans has something to say about this.
Those NC bureaucrats are dangerously deluded. This has nothing to do with the stigmatization of gay men or their marginalization – it has to do with public health.
The Left is trying to make a medical issue into a political issue. It is unconscionable because an innocent person might get AIDS from a homosexual’s blood, latently or manifestly and immediately.
I have nothing against them, but we really shouldn’t put innocent people in danger.
Oh, and I have been donating whole blood for almost 20 years. At first I was worried I might have hemochromatosis, but my iron levels were fine, despite my Celtic DNA. I just kept on donating to help other people.
And by the way, the Red Cross confirmed I had (a) reactive and (b) positive COVID antibodies in my blood in March 2021, which means I caught the Coronavirus. Big deal! It was like the flu.